High-throughput lymphocyte reaction assays take time, skilled scientists, and cutting-edge laboratory instrumentation. Partnering with our specialists for immunogenicity compound screening will help you progress quickly and make informed decisions for the next step of your project.
What is a Mixed Lymphocyte Reaction assay?
The MLR assay is a platform for testing compounds that modify the interaction between an antigen-presenting cell and T cells to activate, deactivate or repolarize the lymphocyte cell response. This assay allows testing the efficacy of therapeutics and other immunomodulators. It also provides critical information for immunological responsiveness for drug safety.
Why should I consider the Mixed Lymphocyte Reaction assay for my project?
The MLR constitutes an in-vitro reactivity test that measures the cell microenvironment reaction to a drug helping to predict how the compound would interact with the body. Drug developers use this assay to test the efficacy of therapeutics that potentially increase, decrease, or repolarize the interaction between T cells and the antigen-presenting cells.
Horizon's standard MLR assay
Our one-way MLR assay enables rapid identification of drugs that regulate T cell activation via allogenic monocyte-derived dendritic cells (Mo-DC). We test your biologics or small molecules at semi-automated high throughput screening in an immune in vitro microenvironment context.
Key advantages:
- Rapid semi-automated and reproducible 384-well high throughput assay
- Applicable for drug safety, immuno-oncology, and autoimmunity applications
- Providing concise and robust quantitative data in as little as 4 weeks
- Highly enriched human primary T cells and Mo-DC from multiple donors to address donor-to-donor variability
Request a quote
Discuss your project with our team
From planning and execution to data analysis, we have the expertise to move your project forward.
Immunology services
Browse immunology assays
Get reliable data from primary immune cells
Read MLR blog
Learn more about MLR assays
This blog covers the basics of MLR assays and how Horizon can support your project
Supporting data
Download App Note
Learn more about the MLR assay and analysis data.
The Mixed Lymphocyte Reaction assay (MLR) relies on the principle that T cells from one donor will respond and proliferate in the presence of antigen-presenting cells (APC) from a different donor, caused by a human leukocyte antigen (HLA) mismatch between unrelated donors. The HLA mismatch stimulates the T cells' immune response, inducing them to proliferate. When proteins on the surface of T cells recognize and bind to companion proteins on other cells, immune checkpoints are activated. These checkpoint pathways engage when T cells bind to a professional APC cell, such as a dendritic cell (DC).
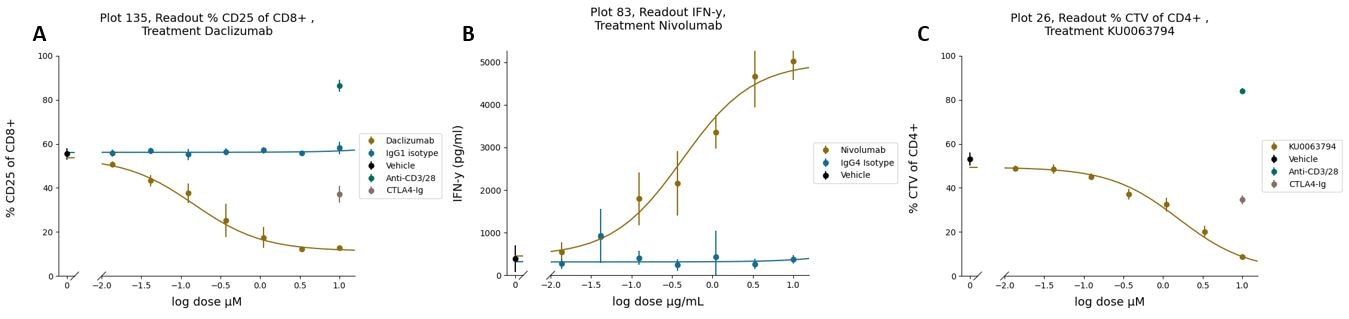
Representative plots of a Maxi-MLR assay: A) Proliferation of allogenic MoDC-challenged CD8+CD25+ T cells in the presence of Daclizumab, as measured by Cell Trace Violet (CTV) staining through flow cytometry. A reduction of the T Cell proliferation is detected compared to IgG1 isotype control. Single donor depicted. Single dose assay controls such as anti-CD3/28 mAb and CTLA4-Ig and vehicle are included as default. B) Interferon gamma release from MoDC-T Cells co-culture treated with Nivolumab. A dose-response increase of INF-g secretion is detected in the co-culture supernatant of by HTRF technology compared with IgG4 isotype. Single donor. C) Percentage of CD4+ T Cells in co-culture with allogeneic MoDCs, in presence of the mTOR inhibitor KU0063794. Single donor depicted. Single dose assay controls such as anti-CD3/28 mAb and CTLA4-Ig and vehicle are included as standard.
