Non-Invasive Prenatal reference material
Non-invasive prenatal testing (NIPT) reference standards support validation, monitoring and troubleshooting of a broad range of prenatal assay methods.
The importance of NIPT reference standards
NIPTs involves screening for fetal abnormalities detected in maternal plasma samples and serve as a non-invasive screening method, compared to invasive Amniocentesis and Chorionic Villus Sampling (CVS), bearing a risk of procedural miscarriage.
In maternal blood, detecting these abnormalities from small amounts of fragmented cell-free DNA (cfDNA) in a high background of maternal cfDNA is a challenge. Conclusively, there is a need to systematically ensure that the NIPT results are consistent and precise in the clinical context worldwide.
Revvity’s new array of reference standards enable the assessment of NIPT test accuracy for Trisomy 21, Trisomy 18 and Trisomy 13 detection.
NIPT reference standard applications
- Enable novel trisomy assay development
- Conduct routine monitoring of assay performance
- Support laboratory external quality assessment (EQA) & proficiency testing
- Intended for research use only
Use extensively validated NIPT reference standards by ddPCR and Vanadis® NIPT platforms
- Improve patient selection for clinical trials
- Assess therapeutic efficacy
- Control end-to-end validation of workflow with cell line derived reference material for close representation of patient samples
- Consider matched maternal-fetal cfDNA based reference material instead of amplified fetal cfDNA
Please note: these NIPT reference materials are only validated with Vanadis platform and ddPCR. We are currently validating with NGS-based platforms.
Vanadis results
The Vanadis® NIPT system is the only NIPT screening platform to enable targeted cfDNA analysis allowing the quantification of chromosomes 21, 18, 13 without PCR, sequencing, microarrays or microfluidics. Instead, 3500 cfDNA fragments per chromosome are captured by highly specific chromosome 21, 18, and 13 probes. After perfect hybridization and ligation of the fragments in both ends of the specific probes, DNA circles including the chromosomal fragments are created. The chromosome fragments in DNA circles are amplified by rolling circle PCR and specific fluorophores are incorporated for each chromosome being analyzed. A proprietary nanofilter plate then captures the labeled DNA molecules for imaging to determine a density score.
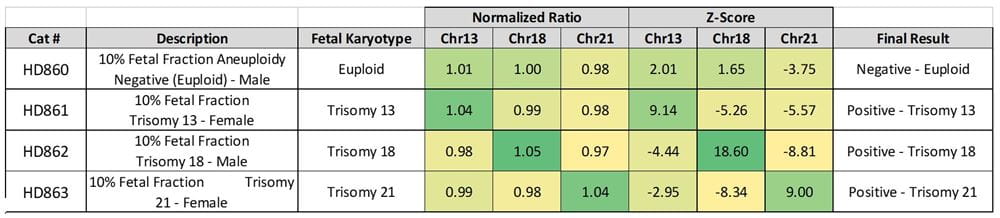
Table showing representative data generated on the Vanadis NIPT platform using Revvity’s NIPT reference product line. Normalized ratios are calculated using the Vanadis NIPT platform imaging data and density scores for chromosomes 13, 18 and 21, which in turn are used to determine Z-scores for each.
 |
 |
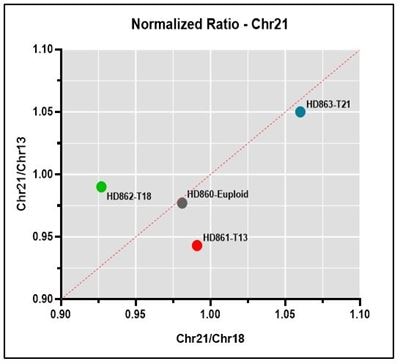 |
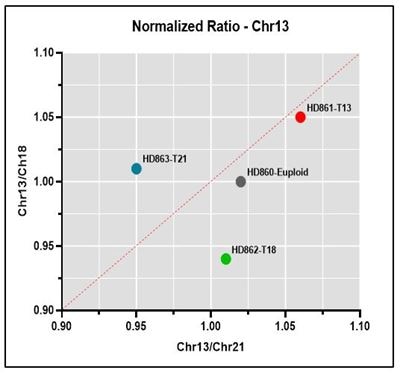
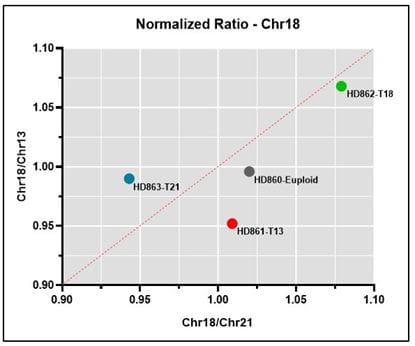
Normalized ratio Representative normalized ratio plots using data generated on the Vanadis NIPT platform and Revvity’s NIPT reference product line. Both the chromosome being analyzed (13, 18 or 21) and euploid data points should lie on or close the diagonal, with the euploid being close to the center of the plot and the chromosome being analyzed in the upper right quadrant if positive for the trisomy.
 |
 |
 |
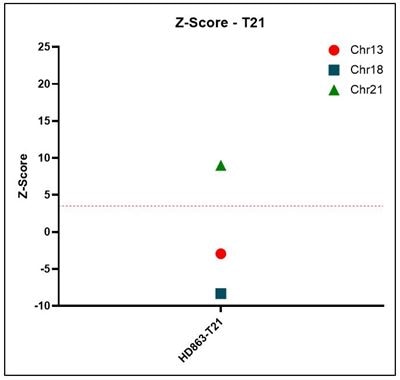 |
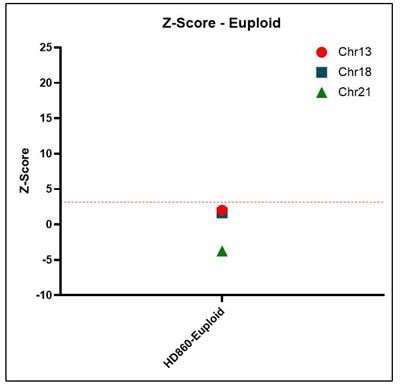
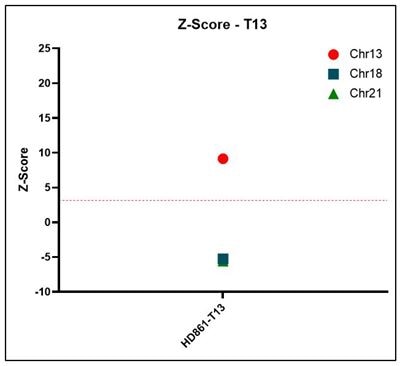
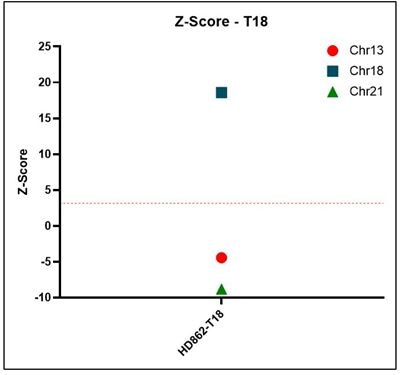
Z-score Representative z-score plots using data generated on the Vanadis NIPT platform and Revvity’s NIPT reference product line. All samples that result in a positive outcome for trisomy 13, 18 or 21 show z-scores above a threshold of 3.15.
Order NIPT Products
These cfDNA reference standards mimic the three most common chromosomal aneuploidies. Each of the product is based on a unique matched maternal-fetal set of cell lines.
Euploid Male-Matched Reference Standard
This control is negative for Trisomy 13, 18 and 21 aneuploidies, providing matched maternal-fetal cfDNA, isolated from a healthy patient.
Helpful resources
Vanadis NIPT System
The Vanadis NIPT system is a cell-free DNA analysis solution intended for chromosome quantification and serves as "A Whole New Way to NIPT"!
Imaging single DNA molecules for high precision NIPT
Dahl, F. et al. Imaging single DNA molecules for high precision NIPT. Sci Rep 8, 4549 (2018).